Every CT site may have 1 or more SC depending on the workload at trial site. And for the control of drugs under investigation.

Role And Responsibility Of Principal Investigator
For protecting the rights safety and welfare of subjects under the investigators care.

Roles and responsibilities of investigator in clinical trials slideshare. Choosing the right CTMS helps address inadequacies on the operational side of research such as clinical trial preparation planning performance and writing for clinical trial monitoring. And for the control of the drugs under investigation. ROLES RESPONSIBILITIES OF STUDY COORDINATOR A clinical research coordinator CRC is responsible for conducting CTs at CT sites according to the protocol ICH-GCP and other regulatory requirements.
If a trial is conducted by a team of individuals at a trial site the investigator is the responsible leader of the team and may be called the principal. A person responsible for the conduct of the clinical trial at a trial site. Uses and Implementation Process of Electronic Data Capture EDC in Clinical Trial Monitoring Service - Pepgra - Clinical Trial Management Systems CTMS are an essential part of every clinical trial.
The clinical investigator is in charge andheld accountable FDA regulations permit sponsors to delegate their responsibilities to contract research organizations CROs but do not permit clinical. The role of a SC in CT is very important. Clinicians working on clinical trials are responsible for their own professional practice as required by their professional codes of conduct.
An Investigator is responsible for ensuring that an investigation is conducted according to the signed investigator statement the investigational plan and applicable regulations. This knowledge is important to ensure. A clinical investigators primary responsibility is to conduct research that contributes to generalizable knowledge while protecting the rights and welfare of human participants1This article part of the Journal of Oncology Practiceseries on attributes of exemplary clinical trial sites2discusses select investigator responsibilities and provides practical advice on how to promote compliance in practice.
ICH GCP E6 R1. The investigatorinstitution should ensure that adequate medical care is provided to a subject for any adverse events including clinically significant laboratory values related to the trial. Responsibilities of a person who conducts a clinical investigation of a drug biological product or medical device an investigator as defined in 21 CFR 3123b and 21 CFR 8123i.
For protecting the rights safety and welfare of the subjects under the investigators care. The functions of the Sponsor-Investigator specifically for investigator-initiated trials include. InvestigatorAn investigator -a person who is responsible forconducting clinical trial at the trail siteIf a trial is conducted by a team of individualsthen the investigator is the responsible leader ofthe team and called the principal investigator.
The roles and functions of the positions and groups involved in conducting clinical trials are provided as guidance for health service organisations to establish or continue to deliver clinical trial services. A To conduct the trial in compliance with GCP b To comply with procedures for data recording c To permit monitoring auditing and inspection d to retain the trial related essential documents until the sponsor informs the investigator these documents are no longer needed The sponsor and the investigatorinstitution. Securing funding for the clinical trial Generating clinical trial documentation such as informed consent protocols as well as submissions for example ethics andor regulatory submissions.
An investigator - is a qualified physician who is responsible for all trial-related medical decisions - must have suitable education training and experience in the field being researched and also have a knowledge of GCP and applicable regulatory requirements - may delegate some of their responsibilities to other individuals. Role and responsibility of principal investigator. Roles involved in conducting clinical trials.
Clinical trial site staff work within and are supported by health service organisations and trial sites to deliver high-quality clinical trials in a safe environment. Results of an investigation. The sponsor should obtain the investigators agreement.
Clinical Investigators are responsible for. 21 CFR 31260 An investigator is responsible for ensuring that an investigation is conducted according to the signed investigator statement the FDAs 1572 form the investigational plan and applicable regulations. Health service organisations or trial sites may also consider these roles and functions to.
DrSurabhi Kirtane Tuesday April 5 2016 1Ref. An investigator shall in accordance with the provisions of. The investigatorinstitution should inform a subject when medical care is needed for intercurrent illnesses of which the investigator becomes aware.
Ensuring that the investigation is conducted according to the general investigational plan protocol and applicable regulations Controlling the drug or device under investigation Obtaining IRB approval prior to enrolling subjects and maintaining continuing approval. CT at site level can be roughly divided into 3 stages. To provide awareness to sponsors and investigators of important responsibilities to adequately conduct clinical trials.
Although the PI may delegate tasks to members of hisher research team she. To ensure patient understand the study.
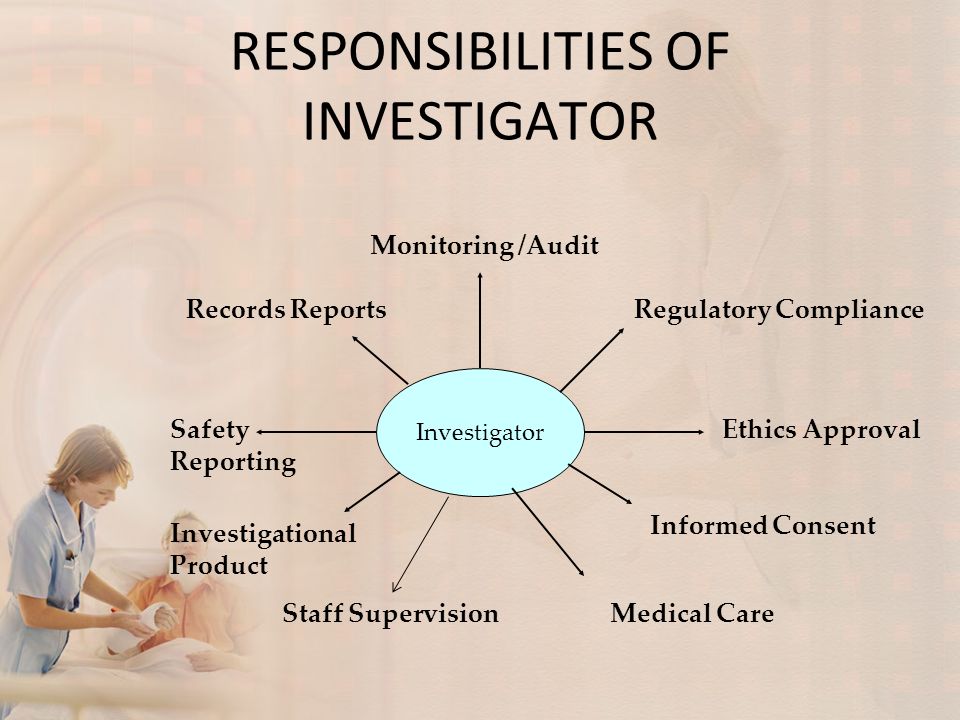
Responsibilities Of Investigator Ppt Video Online Download
2 Sponsor-Investigator An individual who both initiates and conducts alone or with others a clinical trial and under whose immediate direction the investigational product is administered to dispensed to or used by a subject.

Roles and responsibilities of investigator in clinical trials ppt. For studies where there was no enrollment but the IRB-approved you are still required to keep all study documents for 6 years. Role and responsibility of principal investigator. Randomized clinical trials are deemed as a gold standard method for analyzing and evaluating the safety and effectiveness of medical devices or pharmaceutical drugs.
The roles and functions of positions and groups that a health service organisation should establish to deliver clinical trials either investigator-initiated or externally sponsored follow. Device regulations do not require the use of a. Protecting Participants The investigators have a duty to protect the rights safety and welfare of the participants and ensure that informed consent is adequately obtained.
Human Research Ethics Committee HREC HREC Executive Officer. General Clinical Investigator Responsibilities 21 CFR 31260 Ensuring that an investigation is conducted according to the Signed investigator statement Form 1572 Investigational plan. Challenges in the study design conduct and analysis of randomized clinical trials Pepgra - The major steps in conducting a clinical trial study are study design study conduct data analysis and reporting of the findings.
A person responsible for the conduct of the clinical trial at a trial site. And for the control of the drugs under investigation. The individual responsible for the conduct of a clinical study at a site is the principal investigator PI.
Responsibilities of sponsorSelection of Siteand Investigator Sponsor responsibility is to select well qualified trained and experienced investigators for the conduct of trial. Deliver study material documents products and make sure the investigational team understands the protocol and GCP requirements. Trial Initiation visit.
Ensure feasibility in the centre and interest of the investigator. Role of the Investigator. Clinical trial site staff communicate and work with their governing body clinical and non-clinical managers clinicians patients consumers and sponsors to conduct clinical trials within their organisation.
A person responsible for the conduct of the clinical trial at a trial site The investigator is responsible for protecting the integrity health and welfare of the research subjects. Routine Monitoring visit. Prior to randomizing patient if the study is a randomized trialOn going and interactive process between the research team and patient.
The selected investigator should be based at the institutionshospitals having sufficient resources to properly conduct the trial. Clinicians working on clinical trials are responsible for their own professional practice as required by their professional codes of conduct. Allocation of Duties and ResponsibilitiesPrior to initiating a Study the Sponsor should define and allocate all Study related duties and responsibilities.
The Principal Investigator PI is ultimately responsible for assuring compliance with applicable University IRB policies and procedures DHHS Federal Policy Regulations and FDA regulations and for the oversight of the research study and the informed consent process. Do not shred source documents consent forms case report forms IRB documentation until the time period for retaining study documents has passed. While there are many advantages to being a PI for a clinical trial PIs are subject to numerous restrictions designed to ensure patient safety and meaningful study results.
If a trial is conducted by a team of individuals at a trial site the investigator is the responsible leader of the team and may be called the principal. Devices An investigators responsibilities in conducting clinical investigations of a medical device are provided in 21 CFR Part 812 including the requirement that there be a signed agreement between the investigator and sponsor see 21 CFR 81243c4 and 812100. An Investigator is responsible for ensuring that an investigation is conducted according to the signed investigator statement the investigational plan and applicable regulations.
The PI is a model of responsible clinical trial conduct in their field of practice and is responsible for adequately supervising their clinical trial team. The term does not include any person other than an. The sponsor should obtain the investigators agreement.
A To conduct the trial in compliance with GCP b To comply with procedures for data recording c To permit monitoring auditing and inspection d to retain the trial related essential documents until the sponsor informs the investigator these documents are no longer needed The sponsor and the investigatorinstitution. Otherwise specified in a Clinical Trial Agreement. 424 The investigator should ensure that all persons assisting with the trial are adequately informed about the protocol the investigational products and their trial-related duties and functions.
Investigator Responsibilities Regulation and Clinical Trials FDAS 2012 Clinical Investigator Training Course Cynthia F. DrSurabhi Kirtane Tuesday April 5 2016 1Ref. Pre - Trial Monitoring visit.
ICH GCP E6 R1. For protecting the rights safety and welfare of the subjects under the investigators care. RESPONSIBILITY OF AN INVESTIGATOR Informed consent of trial subjectObtain informed consent from patients or parents of minor patients Prior to starting protocol.